Great occasions do not make heroes or cowards; they simply unveil them to the eyes. Silently and imperceptibly, as we wake or sleep, we grow strong or we grow weak, and at last some crisis shows us what we have become. – Brooke Foss Westcott, British Theologian, 1825-1901
BioMarin Pharmaceutical continues on the war path
Two weeks ago, I forewarned that BioMarin Pharmaceutical was headed toward a crisis and last week we discussed the accidental “reply-all” email the CEO sent out revealing the company’s crisis strategy. I could never have predicted this week’s developments.
I have witnessed and studied crises of one sort or another over the last two decades and I have difficulty recalling too many examples of companies handling issues they face as poorly as BioMarin has.
Oh, we had Kenneth Cole tweet a few months ago that the uprisings in Egypt were caused by his Spring collection; and Abercrombie & Fitch has endured several years of criticism after their CEO said that the company only markets to good looking people. But even in tasteless fashion empires, we do not frequently see CEOs go on email rampages in response to public outcry about their company’s behavior.
Supporters of Andrea Sloan have forwarded emails I will share below, and in conversation with Andrea, she discussed with me her feeling of having been mislead by the company’s Chief Medical Officer, who is no longer a licensed doctor.
To catch you up if you have not read previous articles on the situation, Andrea Sloan is an ovarian cancer patient. Her doctors at MD Anderson say that due to her treatment history, traditional, available therapies will no longer be tolerable by her body. BioMarin pharmaceutical has had a drug in trials that the FDA has indicated it will permit Andrea to use if the company will give it to her. BioMarin has promoted this particular drug, BMN673, to investors as the safest and most effective drug of its type. But to Andrea and her doctors, the company says they just don’t know if it is safe enough. Over the last few years, the FDA has allowed over 3,000 patients to use drugs that are not yet approved as, basically, a last resort; while denying only a handful of such requests.
What Not To Do if You Are a CEO
Supporters of Andrea Sloan have used social media and letter writing campaigns to appeal to the company in hope they will allow her and others who face her circumstances a last hope. The letters that I have seen range from heartfelt appeals for moral and ethical behavior, to logic and business reasons it would make sense for the company to grant Andrea compassionate use of their drug.
For a couple weeks, most of the emailed letters Andrea’s supporters sent to the company went unanswered. Over the last few days, though, that changed; and in a somewhat dramatic manner.
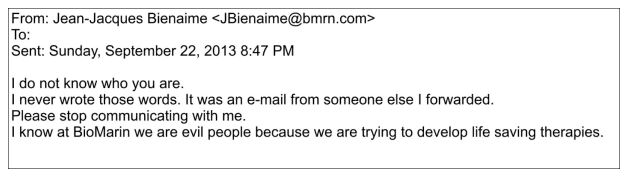
BioMarin’s CEO, Jean-Jacques Bienaime, suddenly started replying to the emailed letters. Far from the measured, careful responses one would expect to come from the CEO of a company, Bienaime resorted to insulting language and at times, unable to come up with his own words describing his perspective, forwarded someone else’s email calling Andrea Sloan “petulant” and “spoiled” as his response.
In Bienaime’s “reply-all” email discussed last week, he laid out two strategies for fighting Andrea: 1. Contradict her doctor’s conclusion that BMN673 is the only drug that has a potential of helping, and 2. Hire a PR firm. Bienaime made good on the aim to contradict Andrea’s doctors in a national media appearance, but BioMarin is apparently still in need of a PR firm; and one which specializes in crisis management at that.
The email exchanges:
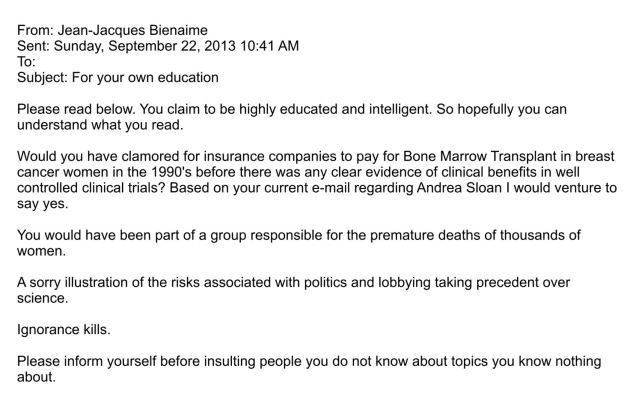
What follows is an email exchange; the first from a supporter of Andrea to Bienaime, the second, his reply to that email:
Beyond it being difficult to understand why his reply is about insurance coverage, which has nothing to do with the situation at hand, his tone is entirely inappropriate. Does BioMarin’s Board of Directors support their CEO’s statements? How do his investors feel? If the company had any type of crisis management plan in place, Bienaime’s responses would not have fit within it.
To another supporter of Andrea, instead of writing his own reply, Bienaime simply forwarded someone else’s words as his response. The email is far too long to paste here in its entirety, but toward its conclusion, it reads:
On social media, supporters of Andrea were livid and a number of them wrote the CEO in complaint of his having endorsed that perspective of Andrea. Here is an excerpt of Bienaime’s reply relevant to those complaints:
No matter what kind of email the CEO of a company gets, this kind of response is never the correct reaction. How does the CEO of a public company think these replies will help his company in any way? And surely he understands that by writing no words of his own in response and simply forwarding someone else’s words instead; those words become his own.
Also of concern: licensing
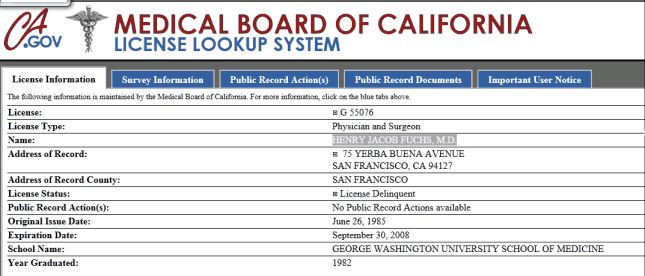
Given that BioMarin’s primary strategy to deny Andrea the drug is to disagree with her doctors at MD Anderson regarding the availability of other options, it came to a surprise to Andrea Sloan that the Chief Medical Officer of the company let his license lapse a few days short of five years ago. According to The Medical Board of California and referencing the date on the image below, if Dr. Fuchs does not renew his license by the end of this month the license will be canceled entirely.
While it is not illegal for Dr. Fuchs to serve BioMarin as its Chief Medical Officer without an active medical license, there has been controversy in other places where problems have occurred in entities which had a non-licensed doctor as its CMO. In this situation, Andrea Sloan feels mislead because she was told that she needed to sign a waiver so that her doctor at MD Anderson could talk to their Dr. and Chief Medical Officer.
Because of communications Dr. Fuchs has had regarding Andrea Sloan’s medical condition and the company’s insistence – amounting to medical advice – that she has other options, at least one Texas Legislator has agreed to file a complaint to the Medical Board of California for there to be an investigation into whether or not Dr. Fuchs actions amount to practicing medicine without a license.
How Does This End?
BioMarin, some argue, is justified in deciding to wait until later in the drug’s trial process before dispensing it outside of trials for any reason. But even setting aside bioethical and moral issues surrounding the ability of a dying patient to have every treatment available that has shown promising results, how can the company justify promoting the safety and efficacy of the drug to investors if they will not stand behind those claims with critically ill patients?
Crisis management can get somewhat complicated at times, but for the most part, common sense dictates the bulk of it. BioMarin, and its CEO in particular, has gone off the rails in their response to the tens of thousands of people who have called on them to provide compassionate use to Andrea Sloan. At this point, the source of the damage that is occurring to the reputation of the company is happening not because of the actions of those contacting the company, but because of the actions of the person who is supposed to be capably guiding the company.
In a situation like this, if I were advising the company as a Crisis Management consultant, I would go directly to the other board members running the company and suggest they sideline the CEO for the duration of the crisis and set forth in a new direction that is less damaging to their mission as a company.
Note: as of publication, the BioMarin PR department has not responded to a request for comment regarding the validity of these emails.
David Holmes, owner of Intrepid Solutions, has over 20 years experience planning for, avoiding, and solving crises in the public policy, political, and private sectors. David is also a professional mediator and has worked in the Texas music scene.







































Deanna L. Kuykendall
September 24, 2013 at 5:04 pm
David, you hit the nail on the head! BioMarin has botched Andrea Sloan’s request for compassionate use from the get-go. It’s been like watching the movie Dumb & Dumber. How can a global pharmaceutical firm get it so wrong? Saying yes to Andrea Sloan’s request should have been an easy decision. I agree with you, the Board of Directors needs to sideline the CEO and CMO and let a crisis management expert get this derailed PR disaster back on track. And before that, tell Andrea Sloan it is going to give her access to the drug her doctors believe is her last hope.
Terry Lynne Stulik
September 24, 2013 at 5:35 pm
BIOMARIN – YOUR CEO NEEDS TO GO! Investors and Board will agree!
BioMarin CEO calls terminal cancer patient a “spoiled petulant brat” https://agbeat.com/business-marketing/public-relations/biomarin-ceo-email-meltdown/ … via @agbeat- GET RID OF BIENAIME, BIOMARIN!
Dorothy Ayer
September 24, 2013 at 10:22 pm
Biomarin wouldn’t need a PR firm if they’d can their spoiled petulant CEO. And THEN put the investors big bucks behind them and help this young woman.
justme
September 24, 2013 at 11:13 pm
I wish someone would interview Andrea’s doctor at MD Anderson again. How did this drug, tested on 28 women, results unpublished/unknown, become her ‘last hope’ for treatment, according to him? Seriously, can her doctor please explain this?
David Holmes
September 25, 2013 at 12:53 pm
The results have been published; you can easily find them in a Google search. And, the CEO and CFO and been going around telling everyone what the results have been. If you would like audio of them saying precisely and scientifically what the results were, I will be happy to send that to you.
Dorothy Ayer
September 25, 2013 at 11:27 am
Food and Drugs Administration a lot of times just rubber stamp test results. Biomarin’s constant red herring and inappropriate comments and responses is bringing FULL attention from the FDA. I hope the FDA searches them with a magnifying glass; and I mean ALL OF THEIR DRUGS THEY HAVE IN DEVELOPMENT. In fact, I would encourage people to start copying their letters they send to Biomarin and forward them to the FDA, requesting a CLOSER look to all of their in-testing drug trials and manufacturing.
MarkBMorrow
September 26, 2013 at 8:10 pm
I guess Mr. Bienaime would sound like a spoiled petulant brat if he was pleading for the chance to save himself from certain death.
JoshGrot
September 27, 2013 at 11:22 am
What’s so particularly sad and frustrating about situations like this is that the public can’t just protest with their wallets and avoid using the company’s products to send a meaningful message back to the company that would change its behavior.
It seems that big pharma companies with de facto monopolies on potentially life saving medications can do just about anything they want as long as they get the drug to market (and it proves to be effective in saving lives).
Shelly Curran
September 30, 2013 at 11:29 am
Problem number one is that he’s French. Problem number two is that he has writers remorse and then lies about it. How is this man a CEO of anything? The woman is dying so who cares if the drug is experimental. She wants a chance. It’s not like her family is going to sue him if she dies, duh!!
David Holmes
October 1, 2013 at 6:53 pm
I don’t completely understand this process, but they don’t pay for the trials – other entities do. So it may be as simple as that a breast cancer focused entity stepped up first.
Ellis Meret
October 3, 2013 at 2:01 pm
When your CEO starts rattling off replies with insulting, rude, ignorant, and condescending language, that’s THE sign your company needs a PR firm. lol. And all those leeches do anyway is distract the public so companies can rub their hands together in the usual way.
Money and power turns people into corrupt scumbags. So does this corporate culture in general it would seem.
jan l
October 4, 2013 at 9:21 am
took the words right out of my mouth! this man has cancer of the heart that has metastasized to his conscience.
Miles Howard
October 9, 2013 at 1:25 pm
Wow. I wonder what would Jonas Salk say?
paperdetective
October 26, 2013 at 7:17 am
I have communicated by email with Biomarin in the last few weeks to try get information on their PARP inhibitor drug for my type of patients.It was terrible. They took their sweet time to respond and first had some kind of $10 an hour ignorant brat answer with canned useless responses and, when I pushed back, they took even more time to respond and it was just a ‘don’t bother us’ brush off again.
Never had that before with any other company, researcher or doctor. Those all responded with informative helpful replies, which I could pass on to fellow patients to help. With some I’m still in touch regularly.
From the trail of BioMarin’s email headers, I can see they have outsourced their communications. Not to their advantage though. It should not be outsourced, since someone who understand the highly technical subject matter and is committed to his company and understands cancer patients and researchers, should answer. Instead we get bureaucratic answers as if we were talking to government insteda of a business with a profit motive.
It makes one wonder if their product is really that promising. If a vendor does not love the customers which he is going to sell his product to, which is how BioMaribn sounds, then why are they in this business at all?
Pam Clark
October 31, 2013 at 7:57 pm
With BioMarin involved (as it should be) with its clinical trials, I suspect that their patient sampling might not be appropriate, and with all of the publicity their data will be highly scrutinized. However, as long as they have control over the initial population sample being tested, they can still present inaccurate data to the FDA. Sloan presents what would be an unknown (but perhaps in this case – a known condition with lots of documentation and tests). I think BioMarin’s CEO is freaking out because their product will not pass muster in the real world.
Their Q1 earnings for 2013 (documented conference call:)
Naglazyme, Q1 2013 sales of $69.4 million
Kuvan, sales of $37.6 million
Pingback: Dead On Arrival: Federal "Compassionate Use" Leaves Little Hope for Dying Patients - Right to Try - National Movement