Biomarin: a study of a public relations nightmare in the making
Today I examine a company who not only serves as an example of the ethical imperative in the structure and design of a business, but I may even be able to forecast a crisis in the making. Most case-studies of crises occur after a crisis has ended or at least after it has begun, but the actions of the pharmaceutical company BioMarin over the last several weeks – while not reaching crisis yet – are moving quickly in the direction of one.
The background of the story is that BioMarin has created a drug called BMN673 that has not yet been approved by the Food and Drug Administration (FDA), but which has shown enormous promise to patients who have gone through trials of the drug. Between 5-10% of ovarian cancer patients develop the cancer due to a genetic mutation called BRCA1. This drug was created for that rarer set of ovarian cancer patients. Creating drugs for rarer diseases is BioMarin’s specialty.
A young attorney in Austin, Texas named Andrea Sloan has the BRCA1 mutation and has fought ovarian cancer off and on since 2007. Traditional treatments are failing at this point, and Andrea’s doctors at MD Anderson view BMN673 as Andrea’s best and possibly last hope.
BioMarin designed this drug for Andrea Sloan, but so far, they will not let her have it. They respond to her requests for the drug by saying that they simply do not have a policy for compassionate use of this drug and it is too early to give out. The FDA, though, has confirmed that it will provide a waiver for Andrea to have access to the drug immediately if the company will only agree to give it to her.
While BioMarin tells Andrea that they want to wait longer to know the drug is safe, the company has issued scientific reports and the CFO has addressed investors with news that the drug has worked well in patient trials and that patients have tolerated the drug very well.
The crisis isn’t over yet
Alert to all crisis management consultants: BioMarin is a company that is not normally public facing yet who may soon face very public scrutiny as a symbol of a broken system
BioMarin’s own “Global Code of Conduct and Business Ethics” states: “It also includes embracing and creating innovative and ethical programs and strategies for expanding patient access to BioMarin products designed to meet unmet medical needs, while at the same time ensuring an appropriate return for our shareholders.”
In pharma-speak, “patient access” often seems to mean “compassionate use” or use as a last resort or under special circumstances. The company has created a crisis precursor for itself by publicly promoting the efficacy of this particular drug to investors while denying its use to a patient who has the exact problem for which it was designed. The one qualification they allow themselves in denying such use in their own rules is “ensuring an appropriate return for our shareholders.” Even though the FDA has indicated it will approve Andrea’s use of the drug, the company has apparently decided that providing the compassionate administration of the drug is somehow not in their financial best interest.
There are ethical obligations the company has to its shareholders, but one should not assume that the shareholder’s only concern is short-term financial. Even if that is the primary concern, the negative public scrutiny that could result from denying this patient the drug could easily harm both the short- and long-term economic health of the company’s publicly traded stock $BMRN.
Contradictory statements and actions
Even if BioMarin’s CFO was not touting the value of BMN673, its reports to the scientific community about the extraordinary potency and the ability for patients to tolerate the treatment provide an ethical obligation for the company to make the drug available to a person who needs it as a last resort.
We have a situation where a drug company has created a drug that is still in trials, but has shown tremendous promise. We have a regulatory body which has shown approval for use of the drug for compassionate use. And we have a patient who has the exact problem for which the drug was created and who understands and accepts the risks inherent in taking a drug that has not yet completed trials.
By what ethical standard can BioMarin continue to deny Andrea Sloan this drug which she must get within the next several days? A moral imperative should supersede a financial one when a person’s life is on the line, but how would BioMarin even face a financial risk in allowing this compassionate use of their innovative drug?
Social media accelerates attention to this issue
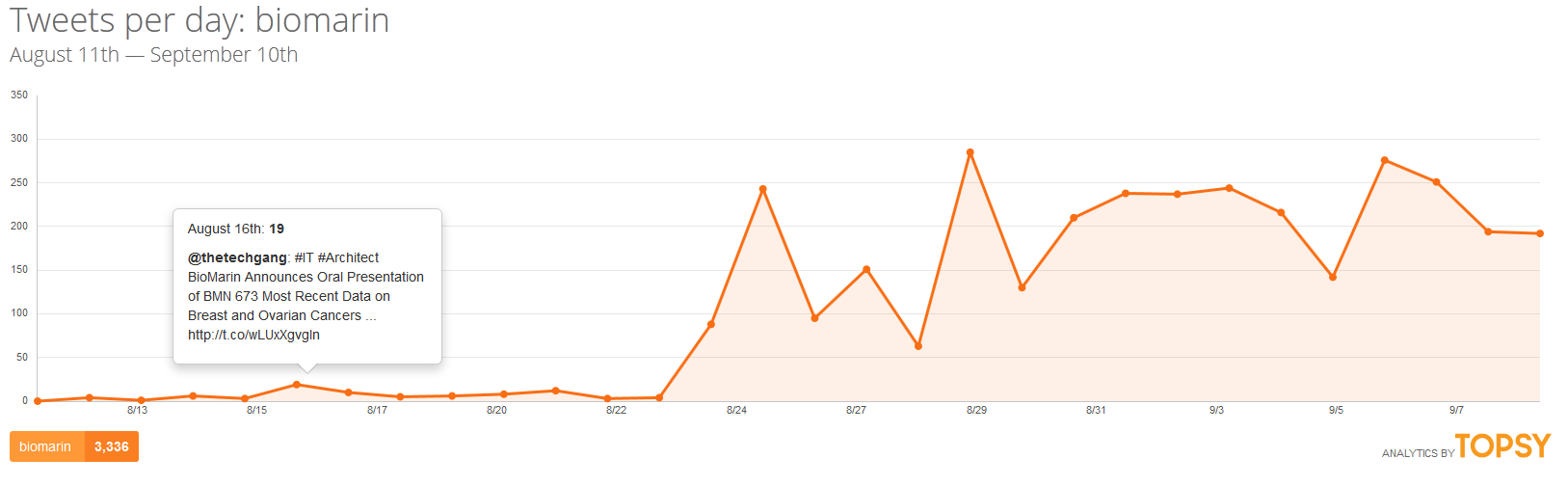
If all of this was happening in a vacuum, there might be little risk of the company facing a crisis. But in today’s atmosphere of social media saturation blending into traditional media coverage, this company which normally only communicates with scientists and investors may find itself as the face of a broken system and be held accountable by the media and a public who might not have the most favorable view of pharmaceutical companies already.
Supporters of @andi_sloan have already initiated a social media campaign that has gained the attention of many people who have never met Andrea Sloan, including a number of celebrities and political figures. A petition has circulated that has already received support of over 30,000 people. CBS This Morning has been the first national media outlet to cover the story and in that story, BioMarin showed that it is not yet ready to face media scrutiny judging from its avoidance of commenting on the issue.
BioMarin can still decide to give Andrea Sloan access to the medication which is sitting on a shelf at the MD Anderson Cancer center where Andrea Sloan receives treatment. If they continue to refuse, however, the company is opening itself to potentially severe scrutiny that could easily rise to the level of a crisis for the company.