Neurotech startup Paradromics is making significant progress with its groundbreaking brain implant, garnering attention from federal regulators. Founded in 2015, the company currently aims to create a device that can assist patients with severe paralysis in regaining their ability to communicate by interpreting neural signals. WOAH.
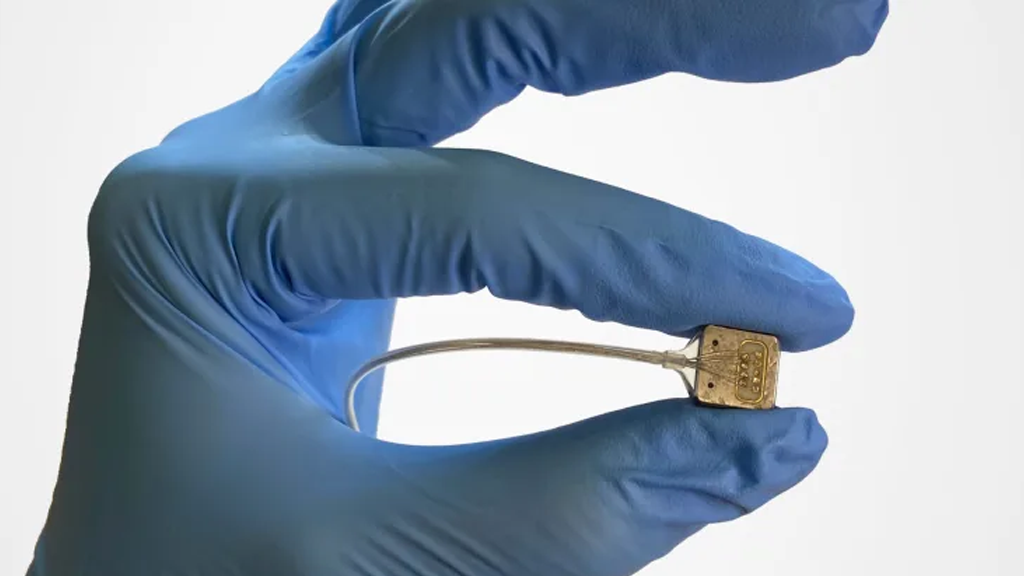
In a recent announcement, Paradromics revealed that its flagship system, the Connexus Direct Data Interface, has received the coveted Breakthrough Device designation from the Food and Drug Administration (FDA). This designation is granted to medical devices that have the potential to improve treatment for debilitating or life-threatening conditions.The Connexus system operates through a transceiver implanted beneath the skin in the chest, enabling it to measure high-quality neural signals.
According to Paradromics CEO Matt Angle, the company’s brain-computer interface (BCI) is engineered to outperform existing BCIs, such as the one developed by rival company Synchron. Angle explained that while the device requires invasive brain surgery, its superior neural signal measurement capabilities allow patients to communicate more effectively and naturally.
Receiving the Breakthrough Device designation offers Paradromics a significant advantage; it facilitates expedited communication between the company and the FDA, potentially accelerating the approval process for future clinical trials. To date, the FDA has granted 32 of these designations in 2023 alone, highlighting the agency’s support for Paradromics’ approach.
Paradromics is currently conducting animal safety trials, and the data from these trials will play a crucial role in determining whether an in-human study can be approved by the FDA. The company aims to initiate its first clinical trial with human patients in the first half of 2024! In a recent funding round, Paradromics secured a hefty $33 million, with Prime Movers Lab leading the investment.
CEO Matt Angle emphasized the exciting advancements in the brain-computer interface field, as numerous companies strive to stand out in an industry estimated to generate billions of dollars in value. While there is great anticipation for the future potential of BCIs, Angle believes that significant positive impact can already be achieved, transforming brain health as we know it today.
Paradromics’ cutting-edge brain implant and its progress towards FDA approval signal a promising breakthrough in neural technology, offering hope to patients with severe paralysis and paving the way for transformative advancements in brain health. How’s that for science?!
Macie LaCau is a passionate writer, herbal educator, and dog enthusiast. She spends most of her time overthinking and watering her tiny tomatoes.